1,2-propanediol
Product name 1,2-propanediol Synonyms propylene glycol
GOST no data CAS 57-55-6 Propylene glycol, also known as 1,2-propanediol is a colorless, odorless, slightly sweetish, viscous, highly hygroscopic liquid. It is fully miscible with water, methanol, ethanol, acetone, diethyl ether, chloroform; boundedly soluble in benzene. Propylene glycol forms azeotropic mixtures with aniline (bp 179.5°C; 43% wt of propylene glycol), o-xylene (135.8°C; 10.0% wt), toluene (110.5°C; 1.5% wt).
Since the molecule of propylene glycol contains a chiral carbon atom C2, it exists in either of two optically active forms (dextrorotatory and sinistrorotatory isomers). Industrial propylene glycol is a racemic mixture. Pure optical isomers may be prepared by hydration of pure (D) or (L) propylene oxide.
Propylene glycol is a diatomic alcohol. It can form mono- and di- ethers and esters being treated with alcohols or acids respectively. It also reacts with alkali metals and alkalis to form corresponding salts (glycolates). 1,2-Propylene glycol dehydrates in the presence of acids or alkalis to form dimethyl-1,4-dioxanes (mixture of isomers). Catalytic dehydratation of 1,2-propanediol at 250°C results in propionic aldehyde.
Propylene glycol reacts with propylene oxide to give the mixture of di-, tri-, tetra- and polypropylene glycols. The yield of products depends on the ratio of reagents and reaction conditions.
1,2-Propanediol is considerably less toxic than ethylene glycol, moreover it is used as a food additive.
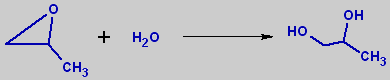
Production.Main industrial method for propylene glycol synthesis is hydration of propylene oxide. Non-catalytic hydration is conducted at 200-220°C, catalytic process proceed at 150-180°C and 1.5-1.8 MPa in presence of ion-exchange resins or small amounts of sulfuric acid or alkali. Final product contains of 20% 1,2-propanediol and 1.5% of dipropylene glycol and small amounts of other polypropylene glycols. Pure propylene glycol can be obtained after rectification.
Uses.
as a moisturizer to maintain moisture in medicines, cosmetics, food, and tobacco products; as a flavoring agent in Angostura and Orange bitters; as a solvent for food colors and flavourings; as a humectant food additive, labeled as E number E1520; as a carrier in fragrance oils; as a food grade antifreeze; in smoke machines to make artificial smoke for use in firefighters training and theatrical productions; in hand sanitizers, antibacterial lotions, and saline solutions as a main ingredient in many cosmetic products, including baby wipes, bubble baths, and shampoos as the primary ingredient in the "Paint" inside a Paintball; as a base ingredient in aircraft deicing fluid and some automobile antifreezes; in cryonics;
vendredi 2 juillet 2010
1,2-propanediol: chemical product info at CHEMINDUSTRY.RU
via chemindustry.ru
Inscription à :
Publier les commentaires (Atom)
Aucun commentaire:
Enregistrer un commentaire